Stability indicating RP-HPLC-UV method development and validation for estimation of Efinaconazole in bulk drug and pharmaceutical formulation
DOI:
https://doi.org/10.5530/gjpb.2025.2.6Keywords:
Efinaconazole, onychomycosis, RP-HPLC, stability indicatingAbstract
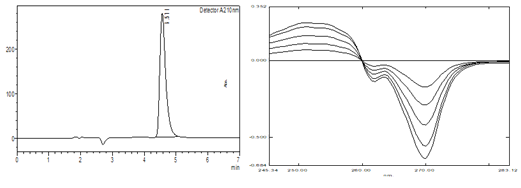
Efinaconazole is a potent triazole antifungal drug for the topical treatment of onychomycosis. In this comprehensive study, we developed a highly effective and reliable stability-indicating RP-HPLC (Reverse Phase High-Performance Liquid Chromatography) method, alongside two advanced UVspectrophotometric techniques—zero-order and first-order derivative methods for the accurate estimation of Efinaconazole. Employing the state-of-the-art Shimadzu model 1800 UV spectrophotometer, we ensured precision and reliability in our measurements. The chromatographic separation was expertly achieved on an Enable C18 column in isocratic mode, utilizing a carefully balanced mixture of methanol and 0.01 M potassium dihydrogen phosphate buffer (pH 5.5) in a 90:10 (v/v) ratio. This was conducted at a flow rate of 2 ml/min, with detection at 210 nm, ensuring optimal performance for our analysis. We rigorously subjected Efinaconazole to five distinct stress conditions, meticulously analyzing the resulting degradation products using the RP-HPLC technique. This allowed us to calculate their percentage recovery with high accuracy. The two UVspectrophotometric methods demonstrated exceptional linearity within the concentration range of 100-500 μg/ml, achieving peak performance with the zero-order method at 261 nm and the first-order derivative method at 270 nm, yielding correlation coefficients of 0.999 and 0.998, respectively. Remarkably, the average recoveries from our recovery studies ranged from 99.44% to 100.42% for the zero-order method and from 99.86% to 100.39% for the first-order derivative method, showcasing the methods' reliability and accuracy. For the RP-HPLC method, we established a linearity range of 25–125 μg/ml, achieving a strong correlation coefficient of 0.998. The retention time for Efinaconazole was consistently recorded at 4.55 min, with recovery rates impressively ranging between 99.8% and 100.08%. This comprehensive validation underscores the robustness and efficacy of our analytical methods for the accurate estimation of Efinaconazole in the treatment of onychomycosis.
Metrics
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Anil Kumar S Manchi, Deepthi Vandkar Jagannath Rao, Lavanya Patil Mulkere Paramesh, Shwetha Manjunatha, Vijaykrishna Chandrashekar Aradhya

This work is licensed under a Creative Commons Attribution 4.0 International License.







